As of December 2023, 10 FDA-approved gene therapies were available. Based on existing development programs, 30-50 additional gene therapies are expected to be FDA-approved by 2030. The impact on treatment outcomes but also the pressure on the budgets of payers is already very strong. How does the uptake look like in some of the countries of Central and Eastern Europe?
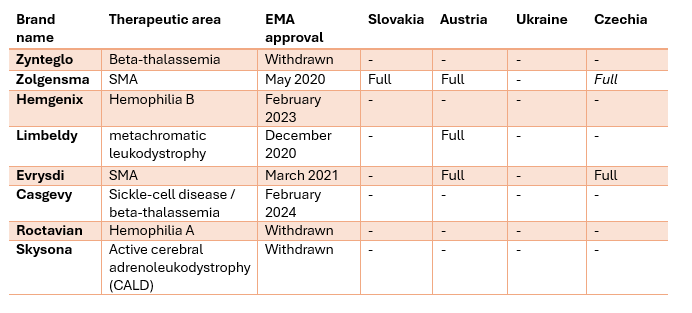
Table 1: Reimbursement status in Slovakia, Austria, Ukraine and Czechia of a set of eight gene therapy brands initially EMA-approved between 2019 and 2024.
Austria reimburses 3 of these in full, 1 each are officially fully reimbursed in Slovakia and Czechia, whilst Zolgensma is not in the official catalogue in Czechia, based on a special agreement between the largest health insurance company VZP and the drug manufacturer Novartis, the VZP has agreed to reimburse the drug. Notably, 3 of the 8 drugs (Zynteglo, Skysona and Roctavian) in our sample have been withdrawn from the EU market by the manufacturers themselves.
Get in touch for more info: info@ambiom.com
Sources:
https://www.ema.europa.eu/en/medicines
https://kategorizacia.mzsr.sk/
https://www.sozialversicherung.at/oeko/views/index.xhtml
https://www.sukl.cz/sukl/seznam-cen-a-uhrad-lp-pzlu-k-1-5-2024
No comments.