Latest in: Uncategorized
Introduction If you asked us (and many other industrial consultants) what is one of the most common commercial strategy mistake we see in otherwise well-positioned early stage companies - without a doubt it would …
The biotech industry in 2024 has seen several high-profile patent disputes that have shaped the landscape of innovation and legal precedent. These cases have centered around cutting-edge technologies such as CRISPR gene editing, mRNA …
As of December 2023, 10 FDA-approved gene therapies were available. Based on existing development programs, 30-50 additional gene therapies are expected to be FDA-approved by 2030. The impact on treatment outcomes but also the …
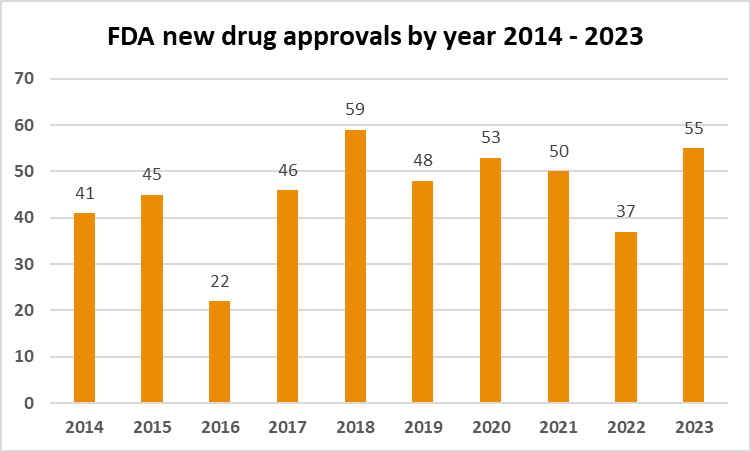
With a focus on addressing a wide spectrum of diseases and conditions, US witnessed the most novel drug approvals in 6 years. Here we provide an overview of the key highlights in treatments that …
Drug development is a complex and resource-intensive process that involves significant investments in research, clinical trials, and regulatory activities. As pharmaceutical companies evaluate the potential of assets they employ various financial metrics. Two commonly …
ambiom · asco · breast cancer · companion · drug development · NGS · pancreatic cancer · rectal cancer · Uncategorized
American Society of Clinical Oncology (ASCO) Annual Meeting was held between the 2nd and 6th June in Chcago, USA. As the last year, here we look at the top 3 highlights we take from …
On 1st of June 2023, the Central & East European Health Policy Network (CEE HPN) organized an oncology workshop in Bratislava, Slovakia. The workshop theme was: ,Discussion on prevention, diagnosis and treatment of oncological …
The new managed entry agreement (MEA) and health technology assessment (HTA) regulations adopted in Ukraine over the course of 2020 and 2021 present significant step forward in improving access to treatment in the country. …
It will soon be 25 years since the first CDx assay came to the market paving the way for the start of the era of personalized treatment. We have discussed the last 25 years …