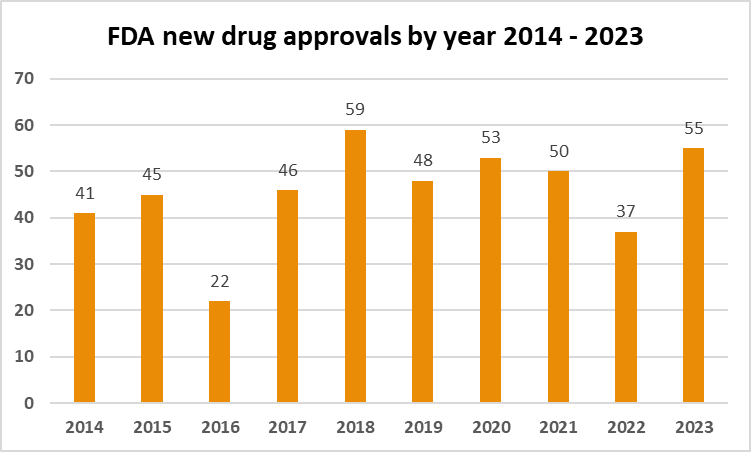
With a focus on addressing a wide spectrum of diseases and conditions, US witnessed the most novel drug approvals in 6 years. Here we provide an overview of the key highlights in treatments that marked the year.
Innovation Across Therapeutic Areas - 27% in oncology indications, 51% orphans
The approvals spanned various therapeutic areas. This included infectious diseases like COVID-19, respiratory syncytial virus (RSV), neurological conditions such as ALS and Alzheimer's disease, opioid use, heart and blood diseases, lung diseases, gastrointestinal conditions, cancers, and women's health issues. Over 27% of all novel approvals were in oncology.
Out of the 55 novel drug approvals, 51% received orphan drug designation (affecting less than 200 000 people in the US). These were targeting rare diseases such as Friedreich's ataxia, candidemia, Rett syndrome, and others. CDER's efforts extended to rare cancers, offering hope to individuals facing conditions like mantle cell lymphoma and nasopharyngeal carcinoma.
First-in-Class - 36% first-in-class approvals
CDER identified 20 of the 55 novel drugs approved (36%) in 2023 as first-in-class. These drugs introduced mechanisms of action different from those of existing therapies, marking a significant leap in medical innovation. Notable examples include Daybue for Rett syndrome, Jesduvroq for anemia caused by chronic kidney disease, Miebo for dry eye disease, Paxlovid as the first oral antiviral pill for COVID-19, Skyclarys for Friedreich's ataxia, Talvey for refractory or relapsed multiple myeloma, Veozah for hot flashes due to menopause, and Xdemvy for Demodex blepharitis.
Get in touch to learn more: info@ambiom.com.
Sources: https://www.fda.gov/drugs/new-drugs-fda-cders-new-molecular-entities-and-new-therapeutic-biological-products/novel-drug-approvals-2023
No comments.