Latest in: ambiom
Introduction If you asked us (and many other industrial consultants) what is one of the most common commercial strategy mistake we see in otherwise well-positioned early stage companies - without a doubt it would …
We spoke in detail about our activities at BeyondBiotech podcast, you can listen to the episode here: https://podcast.labiotech.eu/1995493/episodes/16219760-cracking-the-code-of-biotech-valuations
The biotech industry in 2024 has seen several high-profile patent disputes that have shaped the landscape of innovation and legal precedent. These cases have centered around cutting-edge technologies such as CRISPR gene editing, mRNA …
Earlier this year the FDA has introduced significant regulatory changes for Laboratory Developed Tests (LDTs) to enhance their oversight and ensure higher standards of safety and effectiveness. These changes align LDTs with the regulatory …
On the 20th of February 2024, we have had the opportunity to present our experience and know-how on the online webinar hosted by the BioCentre in Zagreb, to the audience of early-stage life science …
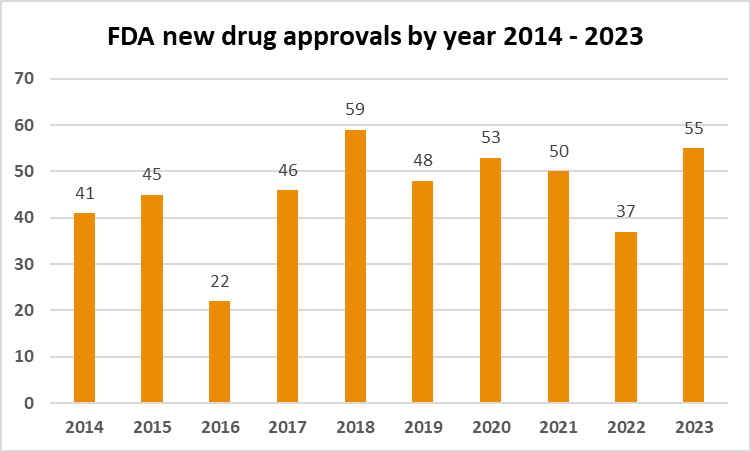
With a focus on addressing a wide spectrum of diseases and conditions, US witnessed the most novel drug approvals in 6 years. Here we provide an overview of the key highlights in treatments that …
Drug development is a complex and resource-intensive process that involves significant investments in research, clinical trials, and regulatory activities. As pharmaceutical companies evaluate the potential of assets they employ various financial metrics. Two commonly …
When a company is in the process of strategizing the development and release of a new product, a notable concern arises, especially in technology-driven fields abundant with patents. The risk is the potential impedance …
The CEE region is home to some of the Europe's most complex clinical centres. Here we took a closer look at the largest ones of them, by bed number. 1. Clinical Centre of Serbia, …
ambiom · asco · breast cancer · companion · drug development · NGS · pancreatic cancer · rectal cancer · Uncategorized
American Society of Clinical Oncology (ASCO) Annual Meeting was held between the 2nd and 6th June in Chcago, USA. As the last year, here we look at the top 3 highlights we take from …
On 1st of June 2023, the Central & East European Health Policy Network (CEE HPN) organized an oncology workshop in Bratislava, Slovakia. The workshop theme was: ,Discussion on prevention, diagnosis and treatment of oncological …
The new managed entry agreement (MEA) and health technology assessment (HTA) regulations adopted in Ukraine over the course of 2020 and 2021 present significant step forward in improving access to treatment in the country. …
UAE has become one of the leading Middle Eastern countries in the biotechnology and pharmaceutical industry due to its heavy investment and development in the sector. In particular, the Dubai Free Zones and the …
In 2019, Novartis’s gene therapy Zolgensma has made news as the „most expensive single-dose drug ever“ with $2.125 million. Just 3 years later, as of November 2022, bluebird bio’s Skysona with the price tag …
Next-generation sequencing (NGS)—a form of DNA sequencing that can examine millions of DNA molecules simultaneously—is proving to be crucial to the successful execution of the concept of precision medicine in oncology but its utility …
The first year with eased-up COVID-19 restrictions, but with significant economic crisis. How did the life science dealmaking fare in the first half of 2022, following the banner year of 2021? Decrease in IPOs, …
The MD Regulation (MDR) 2017/745 is a recent EU directive on medical devices which entered into full force on 26th May 2021 after a transition period of three years, extended in early 2020 for …
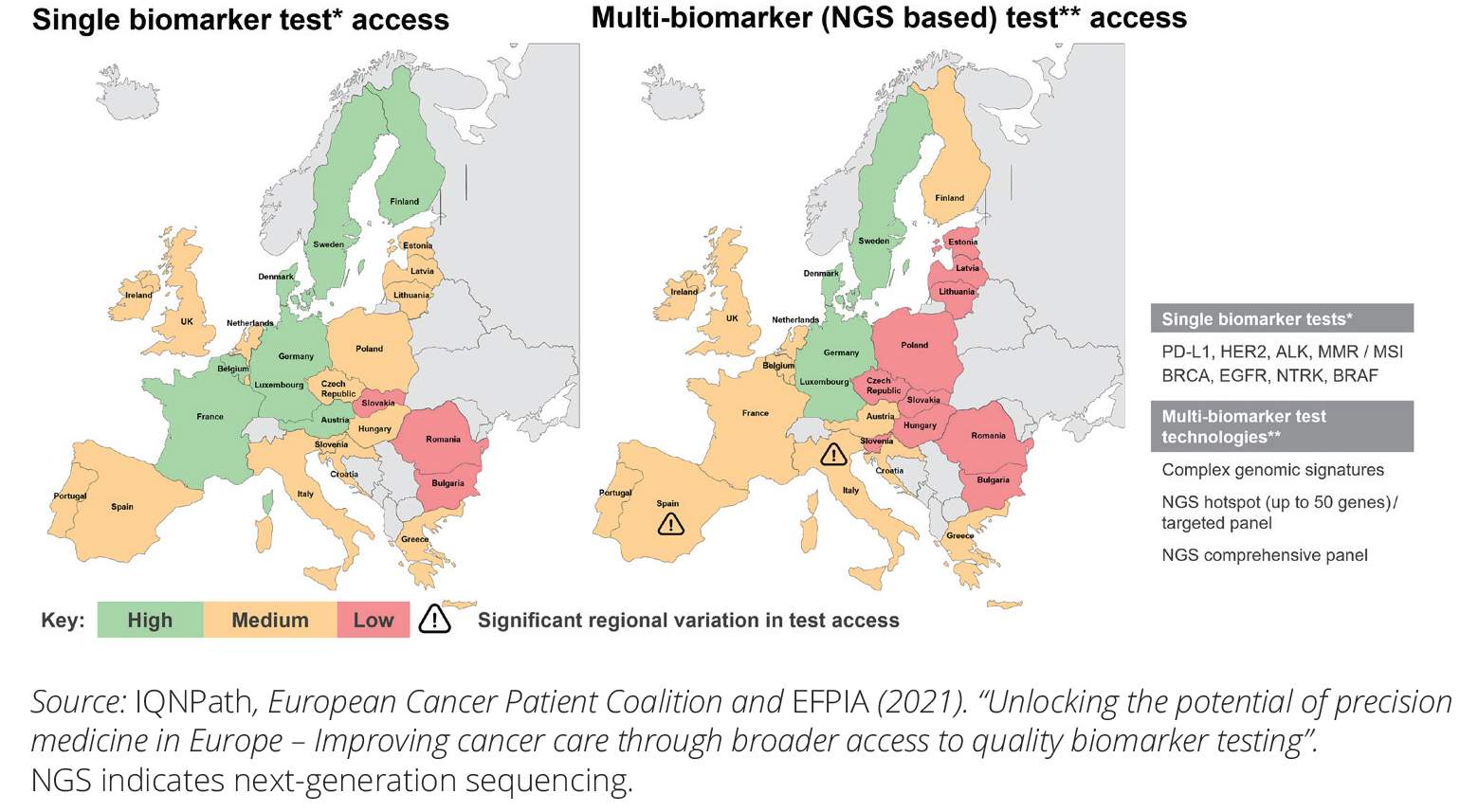
Companion diagnostics (CDx) is used as a companion to a therapeutic agent to determine its applicability (e.g. predict response to therapy, toxicity...) to a specific person. Commonly, companion diagnostics is named as one of …
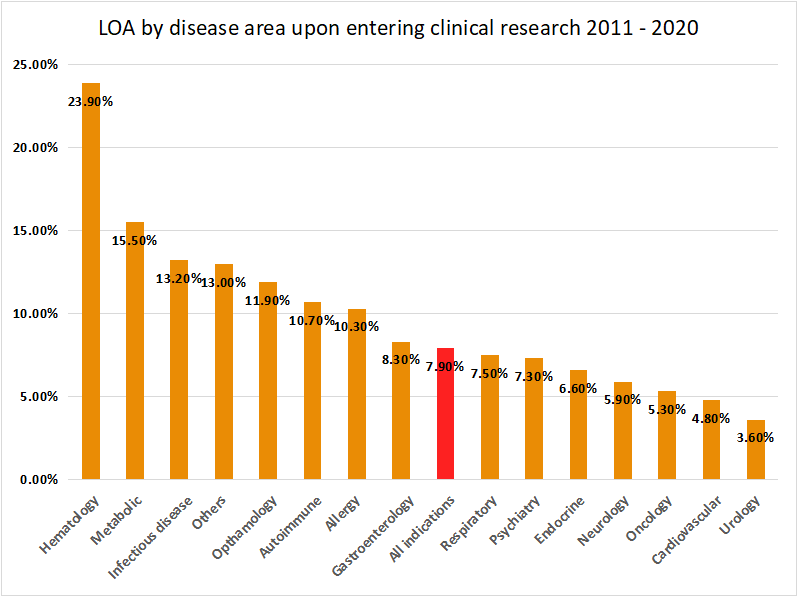
Clinical research success rates of different disease areas have far-reaching implications in the structure of corporate R&D budgets, pharmaceutical asset valuation and, eventually, M&A activity. The report published in February 2021 considers success rates of clinical …