Latest in: drug development
We spoke in detail about our activities at BeyondBiotech podcast, you can listen to the episode here: https://podcast.labiotech.eu/1995493/episodes/16219760-cracking-the-code-of-biotech-valuations
The biotech industry in 2024 has seen several high-profile patent disputes that have shaped the landscape of innovation and legal precedent. These cases have centered around cutting-edge technologies such as CRISPR gene editing, mRNA …
On the 20th of February 2024, we have had the opportunity to present our experience and know-how on the online webinar hosted by the BioCentre in Zagreb, to the audience of early-stage life science …
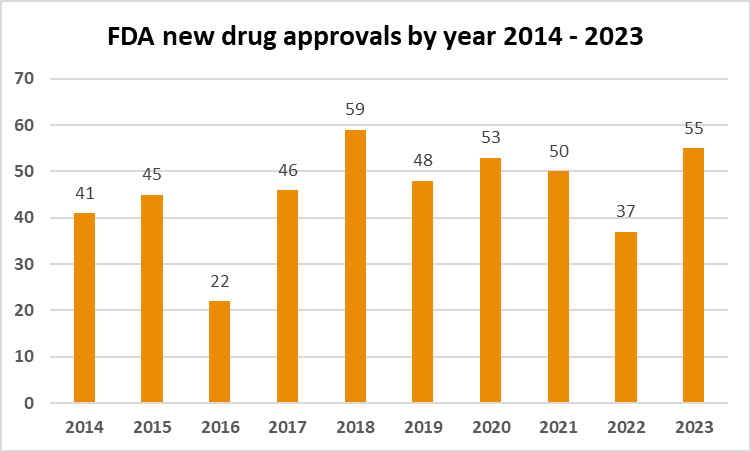
With a focus on addressing a wide spectrum of diseases and conditions, US witnessed the most novel drug approvals in 6 years. Here we provide an overview of the key highlights in treatments that …
Drug development is a complex and resource-intensive process that involves significant investments in research, clinical trials, and regulatory activities. As pharmaceutical companies evaluate the potential of assets they employ various financial metrics. Two commonly …
When a company is in the process of strategizing the development and release of a new product, a notable concern arises, especially in technology-driven fields abundant with patents. The risk is the potential impedance …
ambiom · asco · breast cancer · companion · drug development · NGS · pancreatic cancer · rectal cancer · Uncategorized
American Society of Clinical Oncology (ASCO) Annual Meeting was held between the 2nd and 6th June in Chcago, USA. As the last year, here we look at the top 3 highlights we take from …
UAE has become one of the leading Middle Eastern countries in the biotechnology and pharmaceutical industry due to its heavy investment and development in the sector. In particular, the Dubai Free Zones and the …
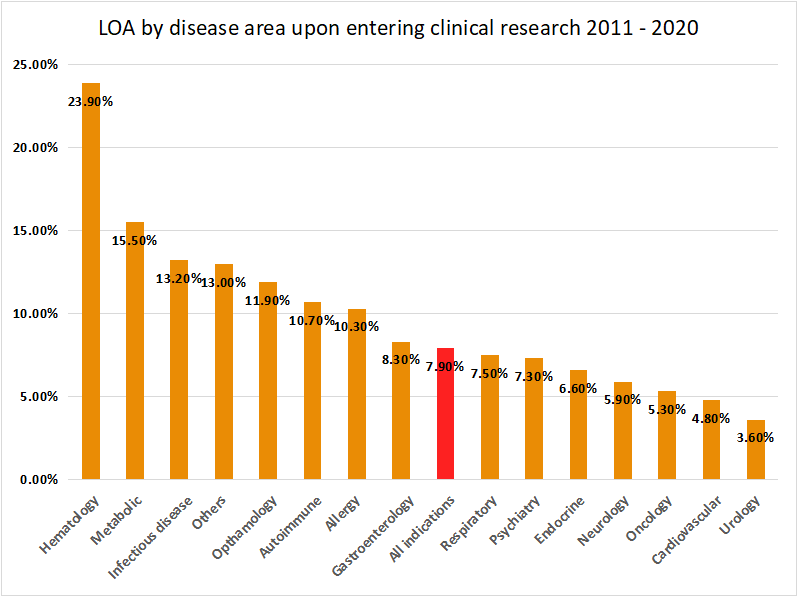
Clinical research success rates of different disease areas have far-reaching implications in the structure of corporate R&D budgets, pharmaceutical asset valuation and, eventually, M&A activity. The report published in February 2021 considers success rates of clinical …