Latest in: clinical research
Introduction If you asked us (and many other industrial consultants) what is one of the most common commercial strategy mistake we see in otherwise well-positioned early stage companies - without a doubt it would …
Drug development is a complex and resource-intensive process that involves significant investments in research, clinical trials, and regulatory activities. As pharmaceutical companies evaluate the potential of assets they employ various financial metrics. Two commonly …
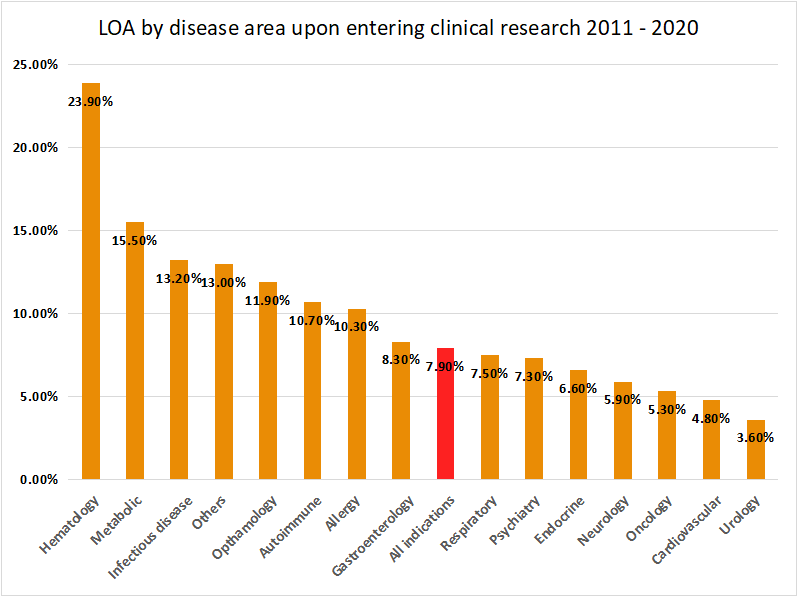
Clinical research success rates of different disease areas have far-reaching implications in the structure of corporate R&D budgets, pharmaceutical asset valuation and, eventually, M&A activity. The report published in February 2021 considers success rates of clinical …