Latest in: precision medicine
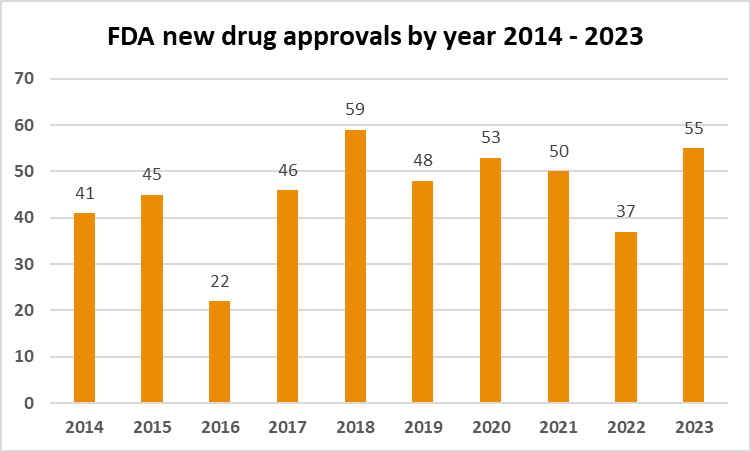
With a focus on addressing a wide spectrum of diseases and conditions, US witnessed the most novel drug approvals in 6 years. Here we provide an overview of the key highlights in treatments that …
On 1st of June 2023, the Central & East European Health Policy Network (CEE HPN) organized an oncology workshop in Bratislava, Slovakia. The workshop theme was: ,Discussion on prevention, diagnosis and treatment of oncological …
The new managed entry agreement (MEA) and health technology assessment (HTA) regulations adopted in Ukraine over the course of 2020 and 2021 present significant step forward in improving access to treatment in the country. …
It will soon be 25 years since the first CDx assay came to the market paving the way for the start of the era of personalized treatment. We have discussed the last 25 years …