Latest in: diagnostics
Earlier this year the FDA has introduced significant regulatory changes for Laboratory Developed Tests (LDTs) to enhance their oversight and ensure higher standards of safety and effectiveness. These changes align LDTs with the regulatory …
On the 20th of February 2024, we have had the opportunity to present our experience and know-how on the online webinar hosted by the BioCentre in Zagreb, to the audience of early-stage life science …
On 1st of June 2023, the Central & East European Health Policy Network (CEE HPN) organized an oncology workshop in Bratislava, Slovakia. The workshop theme was: ,Discussion on prevention, diagnosis and treatment of oncological …
UAE has become one of the leading Middle Eastern countries in the biotechnology and pharmaceutical industry due to its heavy investment and development in the sector. In particular, the Dubai Free Zones and the …
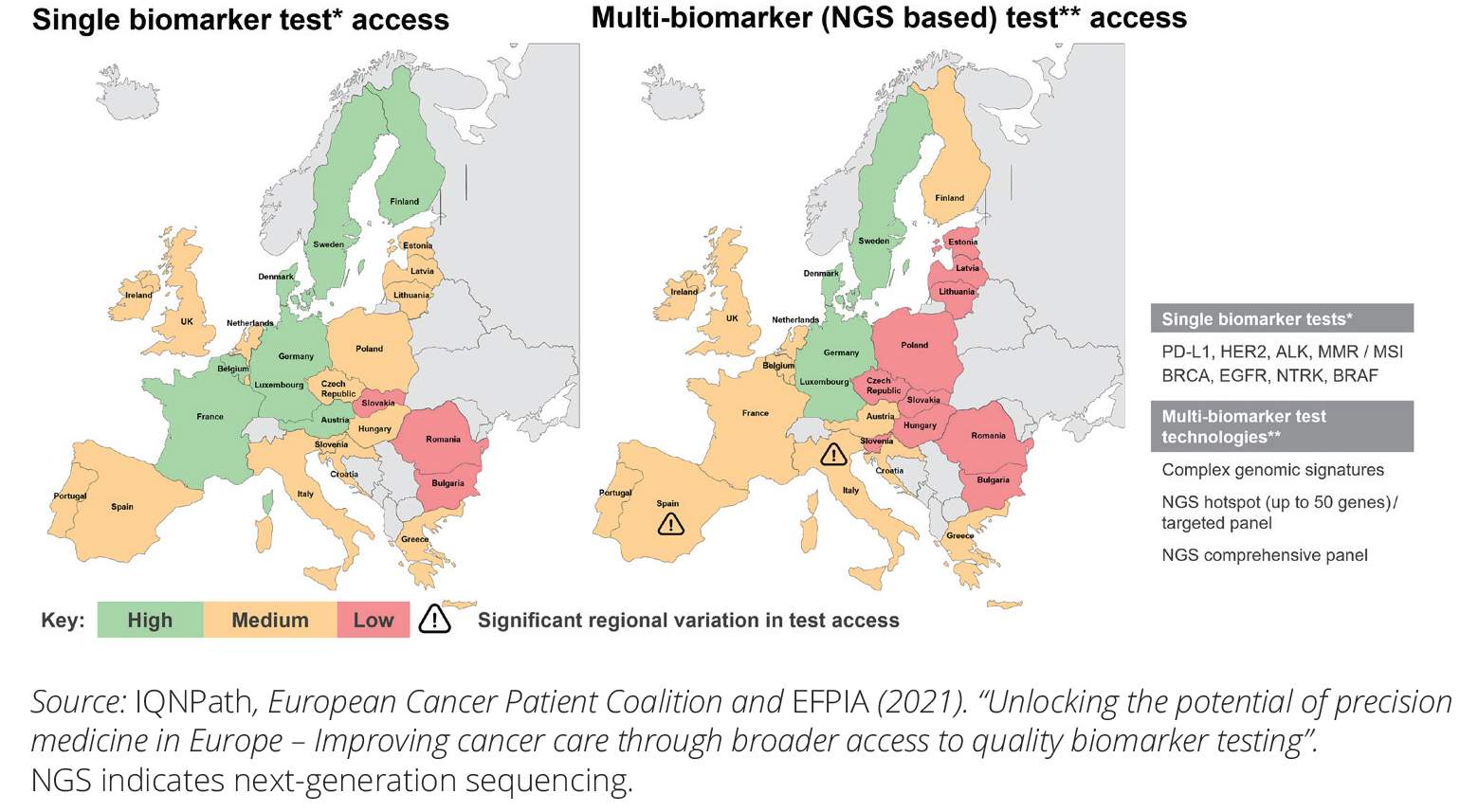
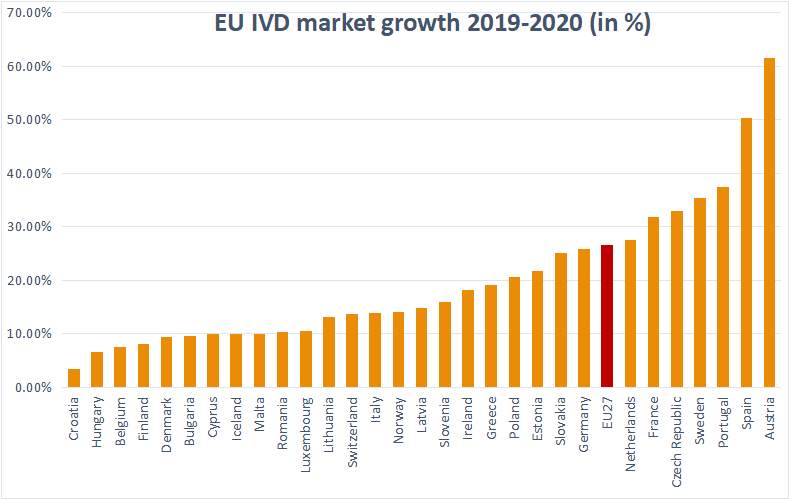
Next-generation sequencing (NGS)—a form of DNA sequencing that can examine millions of DNA molecules simultaneously—is proving to be crucial to the successful execution of the concept of precision medicine in oncology but its utility …
Companion diagnostics (CDx) is used as a companion to a therapeutic agent to determine its applicability (e.g. predict response to therapy, toxicity...) to a specific person. Commonly, companion diagnostics is named as one of …
The Canadian Health Technology Assessment (HTA) agency CADTH released a "Horizon Scan" identifying health tech trends set to significantly impact Canada. These trends aim to leverage data, enhance clinical workflows, and promote accessible healthcare …
The black swan event nobody could have really predicted. 400 million cases around the world and a significant strain on healthcare systems, and the physical and mental capacities of people globally. Could there be a silver …