Latest in: FDA
Introduction If you asked us (and many other industrial consultants) what is one of the most common commercial strategy mistake we see in otherwise well-positioned early stage companies - without a doubt it would …
We spoke in detail about our activities at BeyondBiotech podcast, you can listen to the episode here: https://podcast.labiotech.eu/1995493/episodes/16219760-cracking-the-code-of-biotech-valuations
Earlier this year the FDA has introduced significant regulatory changes for Laboratory Developed Tests (LDTs) to enhance their oversight and ensure higher standards of safety and effectiveness. These changes align LDTs with the regulatory …
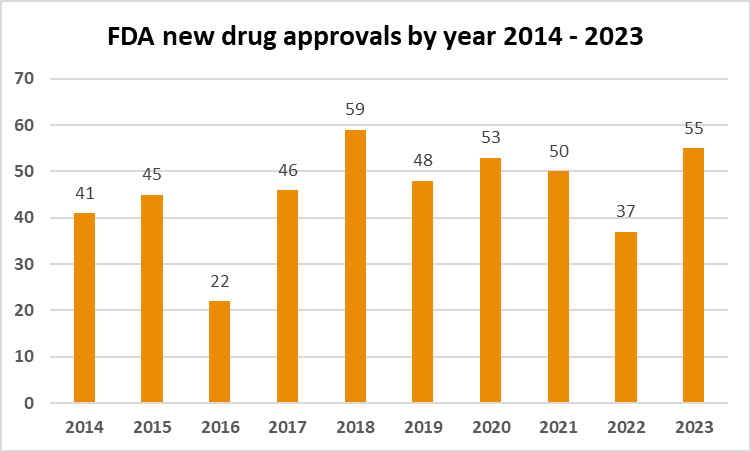
With a focus on addressing a wide spectrum of diseases and conditions, US witnessed the most novel drug approvals in 6 years. Here we provide an overview of the key highlights in treatments that …